
Preparing Murashige-Skoog Media: Step by Step Procedure
As a content and community manager, I leverage my expertise in plant biotechnology, passion for tissue culture, and writing skills to create compelling articles, simplifying intricate scientific concepts, and address your inquiries. As a dedicated science communicator, I strive to spark curiosity and foster a love for science in my audience.


You must know, of all the media, the Murashige-Skoog (MS) medium is one of the most extensively used in plant tissue culture labs, worldwide. So, what is its recipe? What concentration of nutrients is required for the media?
Introduction
Can you imagine in-vitro culturing without using media?
“No way!” Right?
But, why?
The answer to the question is simple, because plants need nutrients for their growth and survival, like all other organisms. In a natural environment, they fulfill their needs by getting it through the atmosphere, soil, and by associating with other organisms.
However, when plants are cultured inside the lab, the support and nutrients for their growth are provided to the plants through media.
In the article “Tissue Culture Medium: Types and 5 Steps of Selection” you can learn about the various types of media used to culture in-vitro plants. You will also learn about the purposes served by different mediums, and how to select the right media for your plant in just 5 steps!
You must know, of all the media, the Murashige-Skoog (MS) medium is one of the most extensively used in plant tissue culture labs, worldwide. So, what is its recipe? What concentration of nutrients is required for the media?
This article will answer all of the above questions, with a short description of components of the media.
You will also find a handy chart that you can keep with you while preparing the media in your lab.
Major Components of MS media
The media involves the following four major components:
- Inorganic nutrient: It includes mineral salts that are important for the growth and development of the plants. It is categorized into two groups: Macronutrients (Calcium, magnesium, nitrogen) and micronutrients (copper, iron, and zinc).
- Organic nutrient: It mainly includes vitamins and amino acids, required for the growth and differentiation of the cultures.
- Growth hormones: It includes auxin, cytokinin, and gibberellins. It is essential for the growth and development of tissues and organs.
- Gelling agents: It includes agar and gelatin. It provides support to the cultures for their establishment.
You can refer to the article “Major Components of Tissue Culture Media” to read more about the components of the media.
Media Preparation
Preparation of stock
1. Micronutrient Stock (100X)
- Take 400 ml double-distilled water in a 1L beaker, then weigh the salts given in the table below and dissolve it in the water.
- Transfer the solution to the 1L volumetric flasks, and make up the volume to 1L. Pipette out 10 ml of the solution to make 1L MS media.

2. Iron Stock (20x)
- Take 80 ml double-distilled water in a 100 ml beaker, weigh the components given in the table and dissolve it completely (in the same order as given in the table).
- Transfer the solution to a volumetric flask of 100 ml and makeup to the final volume.
- Pipette 5 ml of the stock solution for 1L of MS media.

3. Vitamin Stock (100x)
- Take a 100 ml beaker and add 50 ml double-distilled water in it. Weigh the vitamins given in the table below and dissolve it completely in the water.
- Transfer the solution to a volumetric flask of 100 ml and makeup to the final volume.
- Pipette out 1 ml of the vitamin stock solution for 100ml of MS media.
- Add vitamins after the media is autoclaved to protect it from heat degradation. Vitamins can be sterilized by ultrafiltration technique.
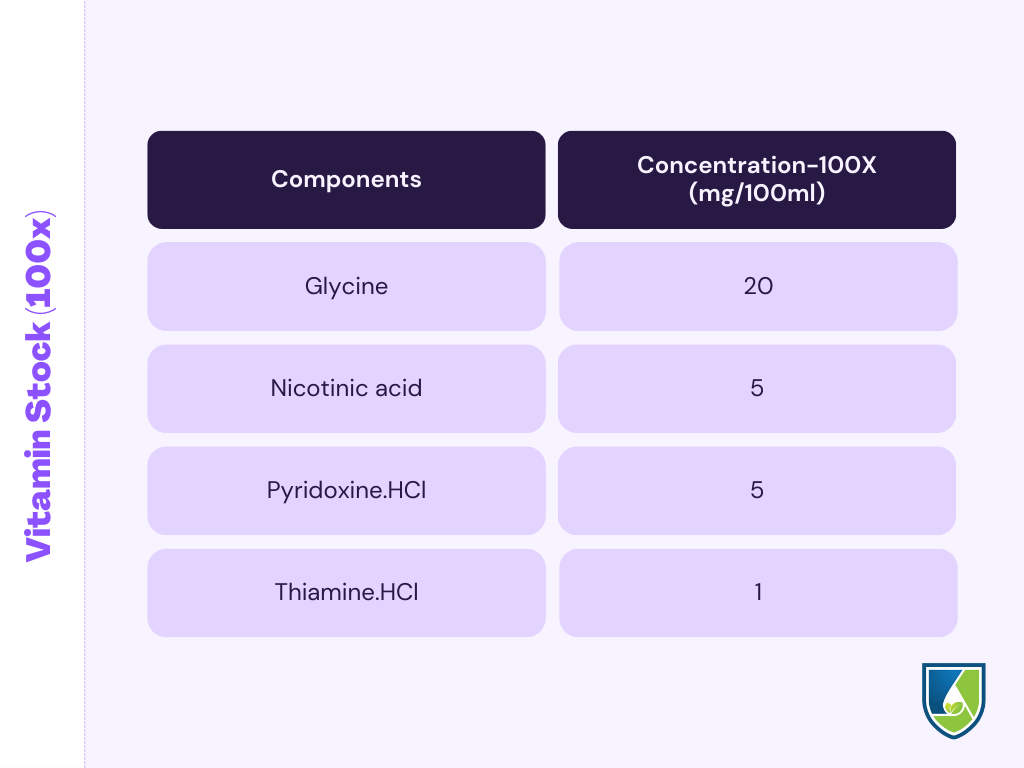
NOTE: Discard the vitamin solution after 30 days.
3. Cytokinin Stock (100X)
- Weigh “10mg kinetin” and dissolve it into a few drops of 1N HCl.
- Add a few ml of double-distilled water to the above solution and transfer it to the 100 ml volumetric flask and makeup to the volume.
- Store the stock in the refrigerator.
- Use 1 ml of the stock for 1L of the MS media.
Steps of the Preparation
Take 400 ml double-distilled water in a 1L beaker. Weigh the macronutrients given in the table below, and dissolve them completely into the water.

- From the prepared stock solutions, pipette out 5ml iron, 10 ml micronutrient, and 1ml kinetin to the 1L beaker of the media.
- Weigh 100 mg Myo-inositol and dissolve it in the previous mixture.
- Weigh 10 mg IAA and dissolve it into a few drops of 1N NaOH. Then, transfer the solution to the previous mixture.
NOTE: The stock of IAA is not prepared because of its oxidative degradation.
- Add 800 ml of double-distilled water in the beaker and adjust the pH of the media to 5.7.
- Transfer the prepared solution to a 1L volumetric flask and make up the final volume to 1L.
- Keep the solution in the refrigerator.
Final steps
- Sterilize all the equipment and glass culture jars used for the tissue culture process.
- Weigh 0.8g of supreme grade agar and 3.0g reagent-grade sucrose and transfer them to 250 ml Erlenmeyer flask.
- Add 100 ml of the stored MS media, in the flask and seal the cap with aluminum foil.
- Sterilize the flask with the media.
- After sterilizing the media for 15-20 minutes, add 1 ml vitamin solution.
- Swirl the flask for the dissolution of the vitamin, agar, and sucrose into the media, before pouring it into the culture bottles.
- Pour the media into culture jars and store them in the refrigerator for 1 hour, before the culturing process.
Now, your culture bottles are ready for the tissue-culture processes.
Here is the handy chart of the MS media recipe for your experiments:
| Components | Concentration for all-100X (mg/L)
except For iron (20X): (mg/100ml) For vitamin: mg/100ml |
|
|
1. Macronutrients |
(NH4)NO3 | 1650 |
| KNO3 | 1900 | |
| CaCl2.2H2O | 440 | |
| MgSO4.7H2O | 370 | |
| KH2PO4 | 170 | |
|
2. Micronutrients |
MnSO4.4H2O | 2230 |
| ZnSO4.4H2O | 860 | |
| H3BO3 | 620 | |
| KI | 83 | |
| Na2MoO4.2H2O | 25 | |
| CuSO4.5H2O | 2.5 | |
| COCl2.6H2O | 2.5 | |
|
3. Iron (20X) (mg/100ml) |
Na2EDTA | 672 |
| FeSO4.7H2O | 556 | |
|
4. Vitamins (100X)(mg/100ml) |
Glycine | 20 |
| Nicotinic acid | 5 | |
| pyridoxine.HCl | 5 | |
| Thiamine.HCl | 1 | |
|
5. Cytokinin (mg/100ml) |
Kinetin | 100 |
| Myo-inositol | 10 | |
| IAA | 10 | |
| Sucrose | 10 | |
| Agar | 30,000 |
Readymade Tissue Culture Media To Increase Your Efficiency
Preparing MS media by adding all the above mentioned chemicals can be a laborsome process. Also, there're higher chances of making errors while adding each components of the media. Sometime you might forget to add a component, while the other time you might weigh and add a wrong concentration of the chemical. Sounds scary, right!
Then imagine if you're preparing to use it for month, what loss will you need to handle after using a wrongly prepared media! Don't even want to imagine, right?
We understand your pain. That's why we provide you a quality and cost-effective MS media that you can easily use in your tissue culture experiments. Just add water and agar to the media, autoclave it, and there you are, ready and prepared to start culturing your pretty plants.
The readymade media not only increase the efficiency and productivity of your lab, but also protect you from facing horrifying situation after using an erroneous media.
Want to get hands-on-experience on media preparation? Plant Cell Technology Can Help!
Plant Cell Technology is helping tissue culturists worldwide by providing unique and world-class products and services that smoothen their process. The PCT Store has MS media, agar, gellan gum, Plant Preservative Mixture (PPM™), culture vessels, Biocoupler™, and masks in its store to facilitate your processes.
We also curate weekly blogs and videos on tissue culture topics. They are all related to tissue culture equipment, practical, tips, and tricks, how-tos, basic concepts, and the latest news. Our YouTube videos teach you exactly how to carry out the tissue culture operation.
And, yes! If you’re stuck at any stage of tissue culture, you can book an appointment with our tissue culture expert. They will help you to solve your query on a video call and give you the right direction to proceed with your experiment.
Occasionally, we also bring you comprehensive master classes led by tissue culture experts, having 15-30 years of experience in the field. The two most frequently conducted classes include Cannabis tissue culture master class and Houseplant and Carnivorous plant tissue culture master class.
Interested in learning more about our master classes and joining your favorite one? Visit this link now!
Blog Categories
View by Level
Popular Blogs

How MSAP Tracks the "Invisible Stress" of Plant Tissue Culture
In plant tissue culture, researchers often encounter a phenomenon where regenerated plants do not resemble the donor plant, despite being genetic...
Read More
Why Labor is Modern Tissue Culture’s Greatest Challenge
Introduction If you look at the numbers, the plant tissue culture industry is on a meteoric rise. We are looking...
Read MoreSubscribe to Our Newsletter
2 comments
This is great… how do I get your products MS
Hello Dear, would you like to assist me how to make 1/2 500mL MS media








Join the conversation
Your email address will not be published. Required fields are marked