Protoplast Culture: Isolation and Culture Methods
As a content and community manager, I leverage my expertise in plant biotechnology, passion for tissue culture, and writing skills to create compelling articles, simplifying intricate scientific concepts, and address your inquiries. As a dedicated science communicator, I strive to spark curiosity and foster a love for science in my audience.


Protoplast is defined as naked plant cells or plant cells without a cell wall. It consists of plasmalemma containing all the other cellular content or components in it. In tissue culture labs it’s used to regenerate a whole plant providing suitable artificial medium and environmental conditions. This process is known as protoplast culture.
Introduction
Protoplast is defined as naked plant cells or plant cells without a cell wall. It consists of plasmalemma containing all the other cellular content or components in it. In tissue culture labs it’s used to regenerate a whole plant providing suitable artificial medium and environmental conditions. This process is known as protoplast culture.
The protoplast term was first introduced by the scientist Hanstein (1880). And, its first isolation was done by Klercker (1892) using a mechanical method. However, the serious efforts in the field of protoplast culture started in 1960, when a scientist named Cocking isolated the protoplast using enzymatic techniques.
Protoplasts of different species are generally fused to produce hybrid plants. The process is known as somatic hybridization (or protoplast fusion). Often a normal protoplast of the plant is also fused with a protoplast without a nucleus (enucleated protoplast) to form a cybrid or cytoplasmic hybrid. The process is known as cybridization.
In protoplast culture, protoplasts isolated from any plant part, including root, shoot, leaves, or embryo, is cultured in an artificial media under artificial conditions favoring cell division and plant regeneration.
This article presents a bring on the isolation techniques of protoplasts and how they are cultured in labs for the regeneration of plants.
Protoplast Isolation and Culture

Protoplast culture involves the following culture events:
- Leaves of the specific plant are taken and sterilized.
- The epidermis layer of the leaf is peeled and the leaf is cut into small segments.
- The leaf segments are put into an enzyme mixture for protoplasts' release.
- Then, the enzyme solution is collected and suspended in a washing tube, which is centrifuged.
- The obtained pellets are resuspended in a sucrose washing medium and centrifuged again.
- The protoplasts are separated at the top of the tube in the form of a band.
- The protoplast bands are suspended in a culture medium.
- The protoplasts are isolated and indued for wall formation.
- After the wall formation, the cells enter into the division phase, forming a clump of a few tissues followed by callus formation.
- Shoots are differentiated in callus and then plantlets are regenerated leading to the forming of a whole plant.

Figure: A detailed schematic diagram of the process of protoplast culture.
Source: Biology Discussion
Protoplast Isolation
Protoplasts can be isolated from different parts of the plants including, roost leaves, stems, microspores, embryos, and fruits. However, among all these sources, the mesophyll tissues of the expanded leaves are the most preferred source of the protoplast isolation.
Protoplasts in labs are isolated mainly using two techniques:
Mechanical Method
In this method, a small piece of the epidermis of the plant is taken and subjected to plasmolysis, causing protoplasts to shrink away from the cell wall. Then the tissue is dissected for the release of the protoplasts.
This method of protoplast isolation is a tedious process and only helps in the isolation of only a few protoplasts. The other limitations are that the viability of the yielded protoplasts is low. However, still, the technique is preferred by some labs because of the deleterious effects of the enzymes used in the enzymatic approach to protoplast isolation.

Enzymatic Method
It’s the most widely used technique of protoplast isolation. In this method, protoplasts are isolated from the source using enzymatic solutions. This technique is faster, efficient, and releases more number of viable protoplasts without any damage.
The plant cell wall is composed of cellulose, hemicellulose, and pectin, thus, the enzymes used for protoplast isolation include cellulase, hemicellulase, and pectinase. The incubation period of protoplast sources in enzymes depends on the type of enzymes used to prepare the solution.
There are two ways of isolating protoplasts using the enzymatic technique. It includes:
- Sequential Method: It involves the use of two enzymes, pectinase, and cellulase, First, pectinase separates cells from middle lamella, and then cellulase separates the protoplast from the rest of the cell wall.
- Simultaneous Method: In this method, both macerozymes and cellulase are used at the same time for complete protoplast isolation.
Protoplast Purification
The separated protoplasts, using mechanical or enzymatic techniques are put under the purification process. Here, all the undigested cells, undigested tissues, and damaged protoplasts are filtered out.
After filtration, the obtained protoplasts are centrifuged, washed, and recovered above Percoll.
Viability of Protoplasts
For successful culturing and increased yield, it’s essential to test the viability of the protoplasts. Some of the methods are mentioned below:
- Fluorescein diacetate (FDA) staining method
- Phenosafranine staining method
- Calcofluor white (CFW) staining method
- Oxygen Uptake measurement technique
- Method Involving the Measurement of Protoplasts’ Photosynthetic Activity
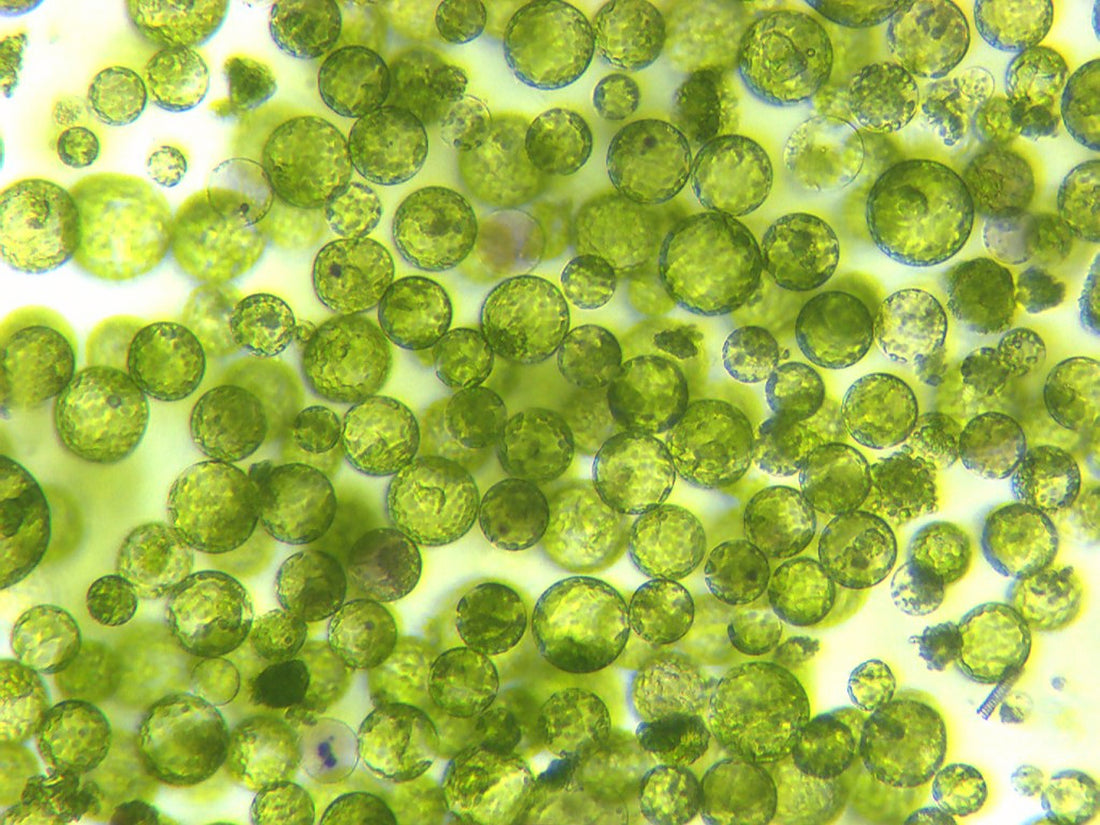
Protoplast Culture Media Types

Image Credit: @robertslothower
The culture medium contains all the nutrients and vitamins required for the growth and development of plants in lab conditions. Mostly MS media is used for such purposes. However, often a modified MS media or B5 media is found to be suitable for the regeneration of plants. It means the formulation of the culture media depends on the species of the plants and their specific growth requirements.
Some special feature of protoplast culture media are given below:
- There should be less iron and zinc and no ammonia in the culture medium.
- For the membrane stability, the calcium concentration should be 2-4 times more compared to that used in normal cell culture media.
- Mainly glucose is the preferred source of carbon, however, a combination of glucose and sucrose can also be used.
- A high auxin/kinetin ratio is required to induce cell divisions, however, a high kinetin/auxin ratio is needed for regeneration.
- Some vitamins can be used as they are used in standard tissue culture media.
Other than the nutritional components, the osmoticum (the chemical which increases the osmotic pressure of the solution) is another factor that needs to be taken care of while preparing the tissue culture media.
For plant regeneration from protoplasts, first, they are required to develop cell walls to proceed to the stage of cell division. Protoplast is cultured either on semi-solid media or liquid media. However, there are also instances where protoplasts are first cultured on liquid media and then transferred to solid media for further development.
Agar Culture
Agar is the most widely used solidifying agent in labs. As much concentration of agar should only be used during the experiments that it forms a soft layer of the gel after being mixed with protoplast suspension.
The protoplasts are cultured on the plate using Bergmann’s cell plating technique. Here, the protoplasts are fixed in a position for cell division and further growth. The agar technique is efficient to prevent the formation of clumps in the cultures.
Liquid culture
It’s the most preferred method for the protoplast culture. Using this technique, the density of the protoplasts can be manipulated and they are easy to dilute and transfer. Moreover, the osmotic pressure of the cultures can also be diluted.

Protoplast Culture Methods
The protoplasts are cultured in labs using the following techniques:
- Feed Layer Technique In this technique protoplast suspension is exposed to X-rays and then plated on agar plates. It’s used to culture protoplasts at low density. Moreover, the feel layer technique is also suitable for the selection of hybrid cells and specific mutants on plates.
- Co-culture Protoplasts This technique is used to culture together protoplasts of two different species. And, you must also note that it can only be used if the protoplasts of the two plant species are morphologically distinct.
- Micro-Drop Culture In this technique, protoplasts are cultures in a special dish, known as Cuprak dishes. The dish has outer and inner chambers. The inner chamber has several walls, in which individual protoplast droplets in nutrient media can be added. The outer chamber contains water to maintain the humidity for the growth of the cultures.
How Plant Cell Technology Is Helping Culturists Worldwide In Their Tissue Culture Application?
Plant Cell Technology is helping tissue culturists around the world by providing unique and world-class products and services that streamlines their tissue culture workflows. It has MS media, agar, gellan gum, Plant Preservative Mixture (PPM), culture vessels, Biocoupler (TM), and masks in its store to facilitate your processes.
And, that’s not it! Plant Cell Technology also offers consultation services to culturists of all sizes that help to get instant solutions to your tissue culture problems.
However, if you're willing to learn directly from an expert, we've got you covered! Our comprehensive tissue culture master classes are designed to learn everything about tissue culture, ranging from theoretical concepts to hands on experience on explant and media preparation, multiplication, rooting, and acclimation of plants. Additionally, it also covers advanced tissue culture techniques, such as shoot apical meristem and synthetic seed production to make you the next pro in the industry.
All our masterclasses are taught by tissue culture experts having experience of 10-30 years in the field. They not only help you learn the tissue culture concepts but also share their personal success and failure stories to learn from them and start your tissue culture journey with a bang.
Seems something long awaited? So, why wait? Sigh up for your choice of tissue culture master class TODAY and excel in your tissue culture game!
Blog Categories
View by Level
Popular Blogs

Is Tissue Culture a Profitable Side Hustle? (Breaking Down the ROI)
Introduction Plant tissue culture (PTC) is capturing the attention of commercial growers, hobbyists, and aspiring entrepreneurs. This innovative method offers...
Read More
Media pH: Why It Matters More Than You Think in Plant Tissue Culture
Introduction Plant tissue culture is a cornerstone technique in modern plant biotechnology, enabling the propagation of plants under sterile and...
Read MoreSubscribe to Our Newsletter
2 comments
excelent work
Good








Join the conversation
Your email address will not be published. Required fields are marked